Comments
Bosstiality t1_j20eq79 wrote
I've not heard of atmospheric water harvesting before! I assume this is for safe drinking water or are there further reasons for harvesting atmospheric water? How does this affect environmental conditions, specifically for things like supercell generation in storm season?
WaterScienceProf OP t1_j20il6k wrote
Yup, AWH provides extremely high quality drinking water. And unlike other water treatment methods, there is no waste stream! It's especially suitable for remote arid regions. The amounts of water removed would be negligible compared to atmospheric flows, but for context, removing water should weaken storms. More info about the work here: https://engineering.purdue.edu/ME/News/2022/atmospheric-water-harvesting-can-we-get-water-out-of-thin-air
[deleted] t1_j20idrx wrote
[deleted]
wasdlmb t1_j22a5x8 wrote
What affects this other than humidity?
WaterScienceProf OP t1_j22oqd4 wrote
Temperature! See our figure from our paper and on wikipedia: https://en.wikipedia.org/wiki/Atmospheric_water_generator#/media/File:Least_work_AWH.png As seen, the energy needs is overlaid on a psychometric chart. The map itself is a yearly average, so it looks worse for regions that have dry seasons, even though AWH may be very easy in their wet seasons.. e.g. Look at Africa North and south of the Congo rainforest, or just South of the Amazon. Also, the map shows a strong influence of Hadley cells, with bands of low and high energy needs: https://en.wikipedia.org/wiki/Hadley_cell
somedave t1_j2349qx wrote
Your graph doesn't go to zero for the humidity ratio. Presumably because the energy required becomes infinite. Does it blow up as the log of this ratio or the inverse?
wasdlmb t1_j22v10x wrote
Thank you. Weather is wild.
The chart makes it look like the energy need mirrors relative humidity, but multiplying relative humidity by a constant wouldn't require high performance computing. Nor even just a defined two input map applied to every point. What was it that took the cluster so much time to compute? Did you have to model the local relative humidity yourself, or is there some more complex relation that I'm not seeing?
WaterScienceProf OP t1_j25u4s8 wrote
The model calculates the Gibbs free energy from removing water vapor, which takes property lookups at each condition. The data intensity comes into play because it's a full year averaged using hourly data, all across the globe.
To the other question: as the humidity goes to zero, the energy needs become extremely high. It also becomes very challenging for practical systems.
something-quirky- t1_j2499r6 wrote
Ah yes, messing with atmosphere. Totally a good idea. I suppose there would be water harvesting companies that would do this? They would probably do it really responsibly :)
WaterScienceProf OP t1_j25vg4q wrote
The atmosphere holds about 12,000 km^2 of water, and the average human needs 8 cups of water per day (the main application of AWH). Thus, it we provided all human drinking water with AWH, it would be about 0.0001%/day.
On a sustainability note, right now the use of river water for drinking can be ecologically damaging, as many water resources are fully exhausted. e.g. the Colorado River and Rio Grande in parts entirely dry up. AWH is a more sustainable source, as the sun provides plenty of continuous evaporation to add more water vapor.
something-quirky- t1_j264xk1 wrote
I think you’re forgetting is that the atmospheric harvesting wouldn’t be evenly distributed across the globe. As your map points out, there would be spots that would be better then other. So you’d expect that the harvesting plants would be not be evenly distributed. So while you may only be extracting some fraction of a percent from the entire globe, you’d be extracting a much larger percentage in the context of the local area. It’s like saying “its okay if i drain this lake because it only takes up .00001% of global fresh water” meanwhile you’ve used up 10% of the LOCAL freshwater. You’re also mischaracterizing the problem. Sure, humans only need 8 cups of water to drink… but you also need to use the toilet, and take a shower, and wash your clothes, and wash your dishes, and cook etc. And you can’t just pump in dirty water for everything that isn’t for drinking. It’s just passing the buck is what it is, and I’d be willing to bet that the resulting weather patterns from this practice would be just as devastating as draining the rivers and streams.
WaterScienceProf OP t1_j2782pb wrote
Water in the atmosphere is a near infinite resource. It stays up on average only 8-10 days, being continuously regenerated by the sun. I don’t mean to be dismissive, but the amount of water in the atmosphere dwarfs currently used freshwater sources by orders of magnitude. And unlike other methods, it doesn’t produce wastestreams, which can be ecologically damaging especially if said wastewater is salty and far from an ocean.
When we pump in dirty water for things besides drinking, it’s called greywater reuse, and is actually far more widespread than AWH.
The real concerns for AWH are around its energy intensity, which is many times that of conventional sources- as a result it’s likely not economically viable for use beyond ultra pure water. And if it’s not powered by renewables it may not be sustainable. And renewable power is still resource intensive to create. You are right to criticize it, but you focused on the wrong issue!
Sources: https://hess.copernicus.org/articles/21/779/2017/hess-21-779-2017.html https://greywateraction.org/greywater-reuse/
nslenders t1_j218swr wrote
those energy values seem low to me. or did i bork my conversion somewhere?
3600Kj/kg would be 1kWh/L --> 180Kj would be 50Wh/L
i would think it would take more than 50w for an hour to get a liter of water
WaterScienceProf OP t1_j21gbq1 wrote
It conveys the thermodynamic minimum possible, not what practical technologies can achieve. Available approaches are still emerging technologies, and may need an order of magnitude or more energy to work. For context, the minimum energy for seawater desalination is ~1 Wh /L.
HardCounter t1_j23jocj wrote
How are you going from 1kWh/L to 50Wh/L? You aren't reducing the amount of water harvested, you're just reducing the power for some reason. 50Wh would be 1/20L with a direct conversion.
Am i missing something?
nslenders t1_j23mb4a wrote
3600Kj is 1Kwh. And 1kg is 1L. But the graph shows values from 0Kj/L till 280 Kj/L
Since 3600Kj is not on the chart. Taking a value that I can easily divide, being 180Kj/L. Would give me 1/20th of that 1Kwh. Or 50Wh/L
The map has values higher and lower, but 180Kj/L looked like a reasonable middle ground to calculate. Taking 1,5x our 180Kj/L would give 270Kj/L, or almost the top values. And is still only 1,5x 50Wh/L or 75Wh/L.
HardCounter t1_j23nv2c wrote
I guess i don't know what you're trying to achieve.
Pick a number between 1 and 280 and it's on the chart somewhere, no math required. 50kJ/kG is on there somewhere, so is 83.4kJ/kG since it seems to be a continuous scale. 280kJ/kG or so seems to be the maximum for Earth. I'm not sure why 3600kJ/kG entered your mind, or what planet that would apply to.
nslenders t1_j23oy44 wrote
My mind does not think in Kj. But if u tell me something uses as much energy as a 50W lightbulb for 1h. I know what that means.
I do know that 3600Kj equals 1Kwh. So I used that as an intermediate step to do the conversion. It does not have to be a relevant value on the map.
The reason I found the values low. Is that I have a big dehumidifier that uses 2000W. But it does not give me 40L every hour. But OP already explained that these are theoretical minimum values.
HardCounter t1_j23rmj8 wrote
2000W? Is it industrial strength? You'd probably get a lot more water if you put the dehumidifier outside, though.
> Portable dehumidifiers typically consume between 30 and 50 watts while whole-home dehumidifiers can use up to 250 watts per hour.
https://www.perchenergy.com/energy-calculators/dehumidifier-electricity-usage-cost-to-run
I also found this:
> On average, a home dehumidifier collects five gallons of water per day.
That's about 19 liters per day inside an already dehumidified home. That comes out to about 315 watts per liter in a relatively low (30-50%) humidity environment. That's not too bad.
I should get a dehumidifier in case of zombie apocalypse.
Fhotaku t1_j287bsz wrote
I did the legwork for a phd project that involved comparing indoor dehumidifiers efficacy on AWC in a desert. The cost varied wildly, in the 10c-4$/liter range, but that also included tests at 5% humidity and 120F outside, and others while it was raining.
Most places could use active systems like this for cheaper and cleaner water than they're currently getting. I will mention though, the refrigeration based dehumidifier collected every damn particulate in the air with it. It definitely needed filtration. Even moreso, this water is salt-free, and you can't live off that. These are costs to consider when using AWC.
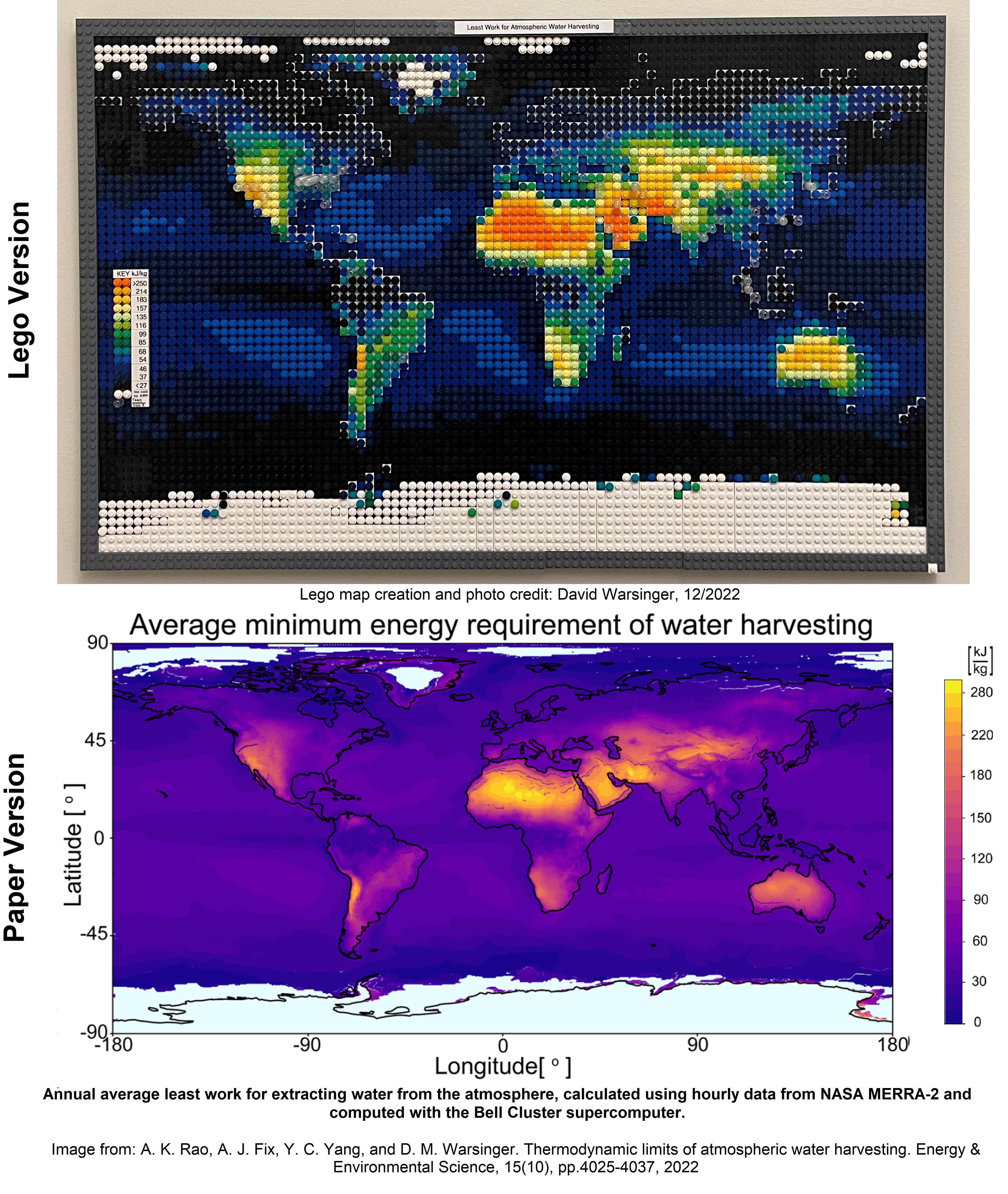
WaterScienceProf OP t1_j209741 wrote
This lego map was created from figure 3a of our paper on Thermodynamic limits of Atmospheric Water Harvesting (link in the title). The original figure used hourly data and took weeks of run time on the Bell Cluster supercomputer to process the calculation.
Anyone can use our code on github to convert an image to the correct size, apply bins for colors, and visualize it before buying your legos: https://github.com/arao53/awh-limits/blob/main/lego_maps/warsinger_lego_maps.ipynb
More details about how to create your own Lego figures are on our post on Lego Education: https://www.reddit.com/r/LegoEducation/comments/zubefd/how_to_make_scientific_graphics_out_of_legos/